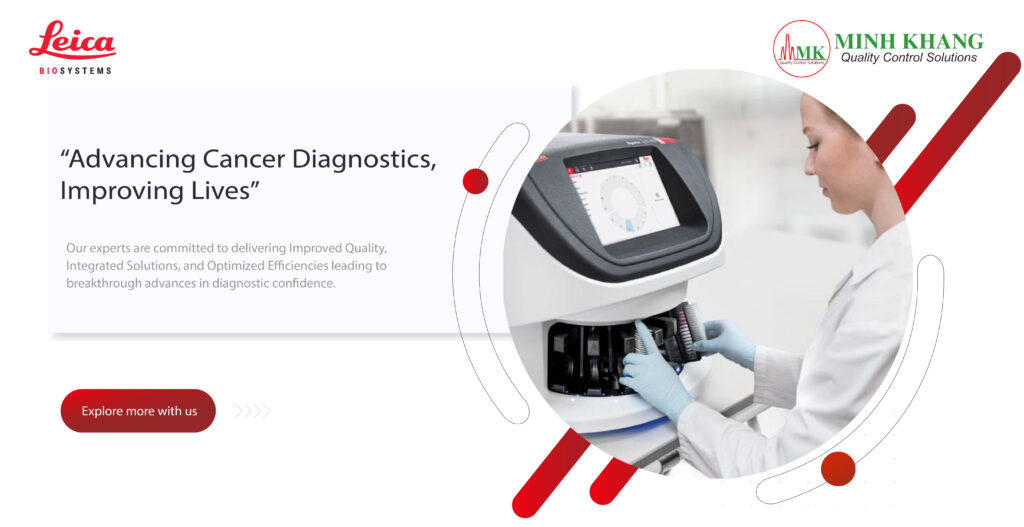
FEATURES
- Automated, reliable, and objective microbial detection with built-in quality control
- Easy-to-use for a streamlined workflow
- Ready to use media bottles
- 21 CFR Part 11-compliant data management system
- Non-destructive sterility testing for subsequent identification
MAIN BENEFITS
- Modular system customized to the capacity of your sterility testing needs
- Real-time results that allow for early interventions
- LIMS compatibility for compliant data tracking
- Globally recognized by the industry and pharmacopoeias as an alternative sterility testing method

















 VI
VI